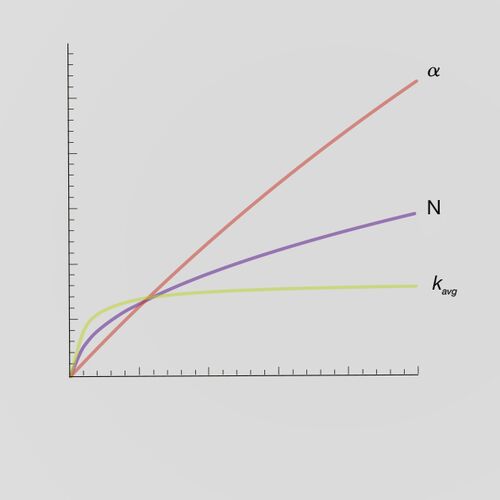
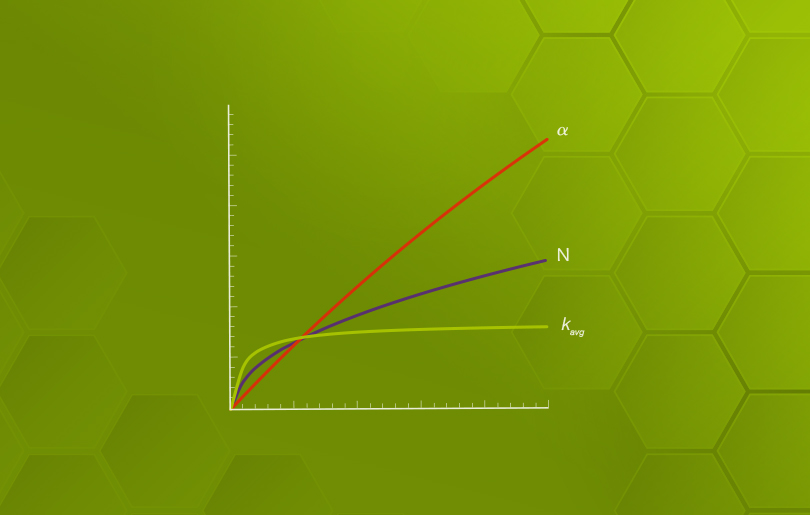
Advantages
- Extended pH range 2 - 10
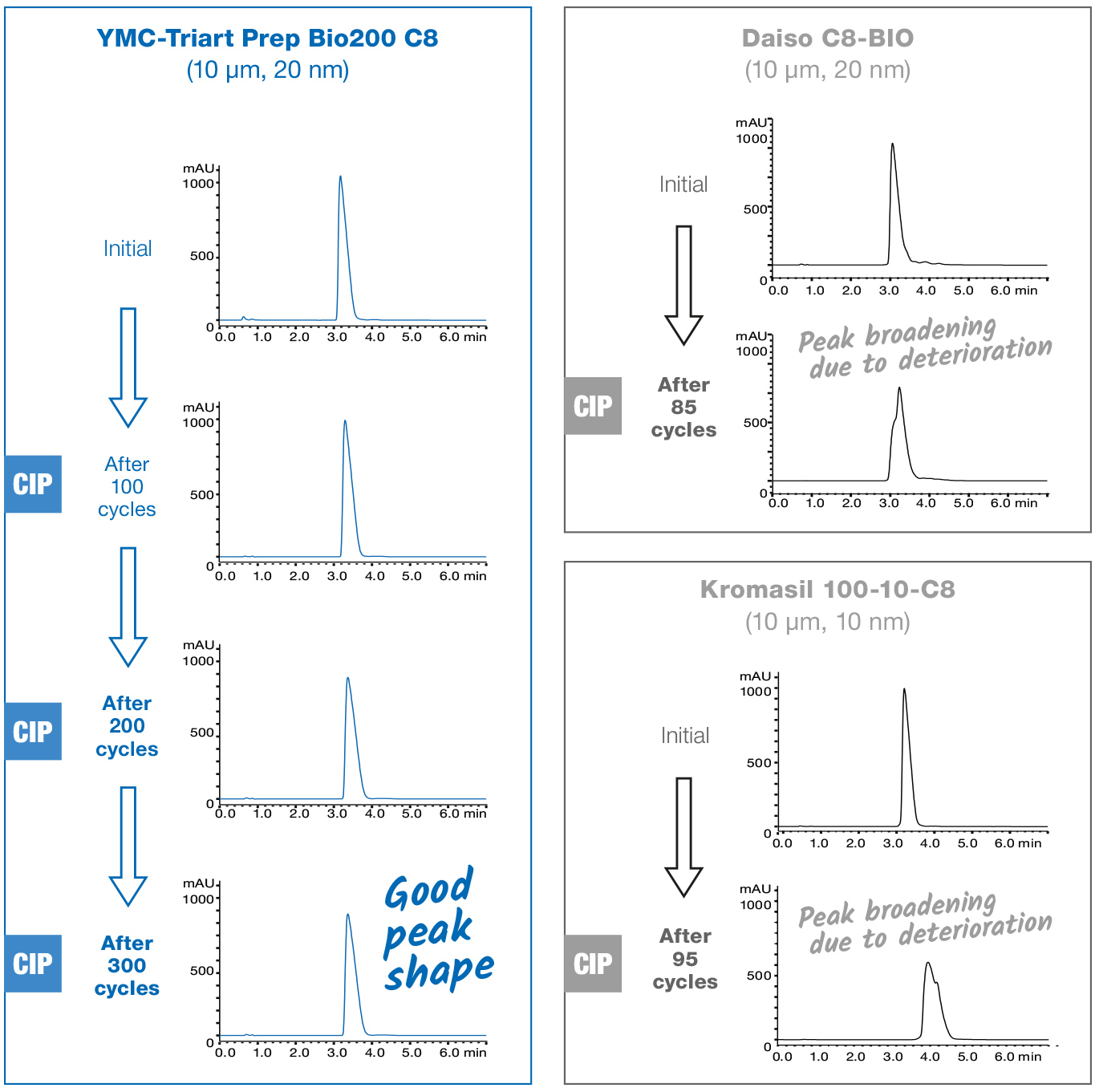
- Alkaline CIP procedures
- Flexibility in method development
- Longer lifetimes
- Improved loadability
- Compatible with 100% aqueous conditions
Available Stationary Phases:
"Almac has been working with YMC and APEX Scientific on various projects for over 5 years – from mg impurity isolation to multi-kg purification and have found the level of technical support and guidance exemplary when using their products. This has ranged from advice on packing large DAC columns to the best media for a particular separation. Furthermore, YMC has presented on a number of different topics at Almac, which has increased our toolbox of purification technologies now regularly utilised in-house."
Steve McIntyre, Almac Group Ltd. (UK)
| YMC-Triart Prep C18-S | YMC-Triart Prep C8-S | YMC-Triart Prep Bio200 C8 | YMC-Triart Prep Phenyl-S | YMC-Triart Prep C4-S | |
| Base Material | organic / inorganic hybrid silica | ||||
| Modification | C18 | C8 | C8 | phenyl | C4 |
| Particle Size [µm] | 7, 10, 15, 20 | 10, 15, 20 | 10 | 10 | 10 |
| Pore Size [nm] | 12 | 12 | 20 | 12 | 12 |
| Surface Area [m²/g] | 360 | 360 | proprietary | 360 | 360 |
| Bonding Type | trifunctional | ||||
| Endcapping | yes | ||||
| pH Range | 2.0 - 10.0 | ||||
| 100% Aqueous Eluents | yes | - | - | yes | - |
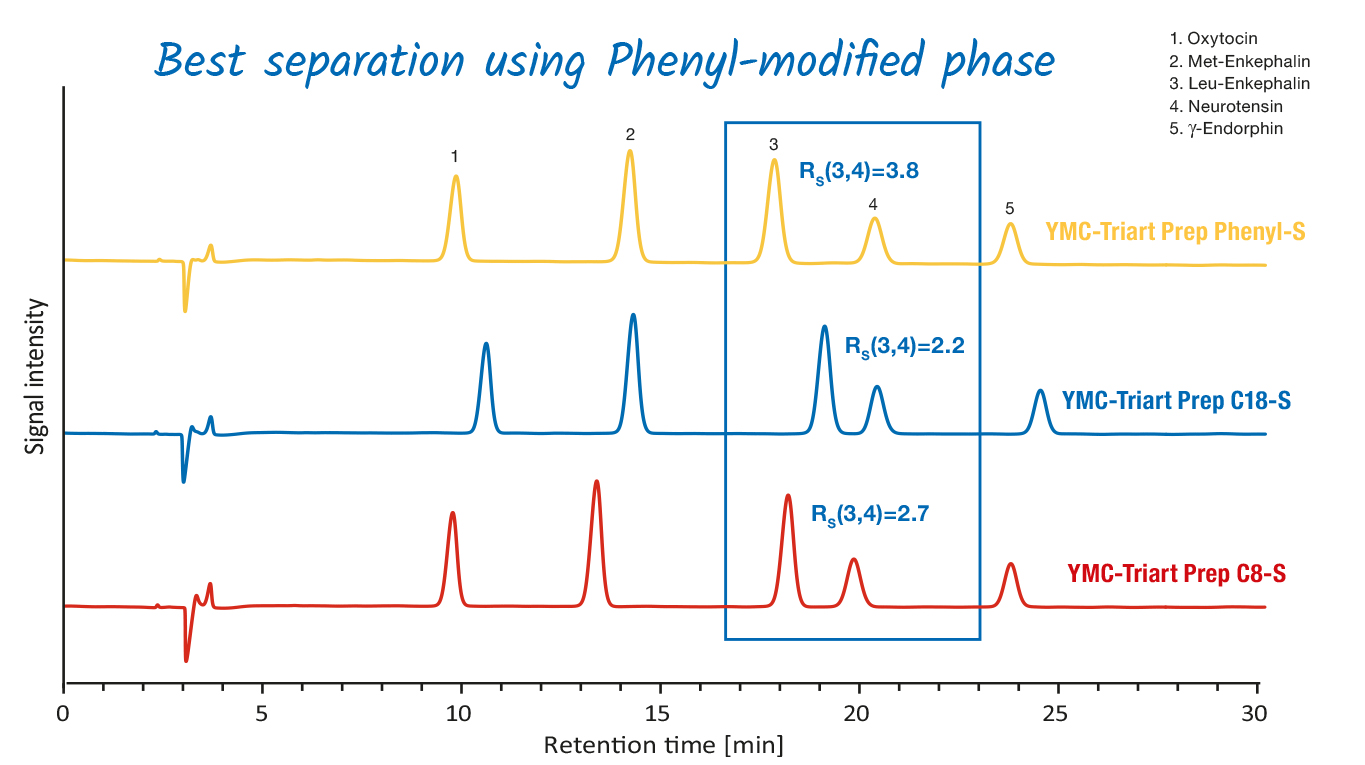
YMC-Triart Prep Phases overview
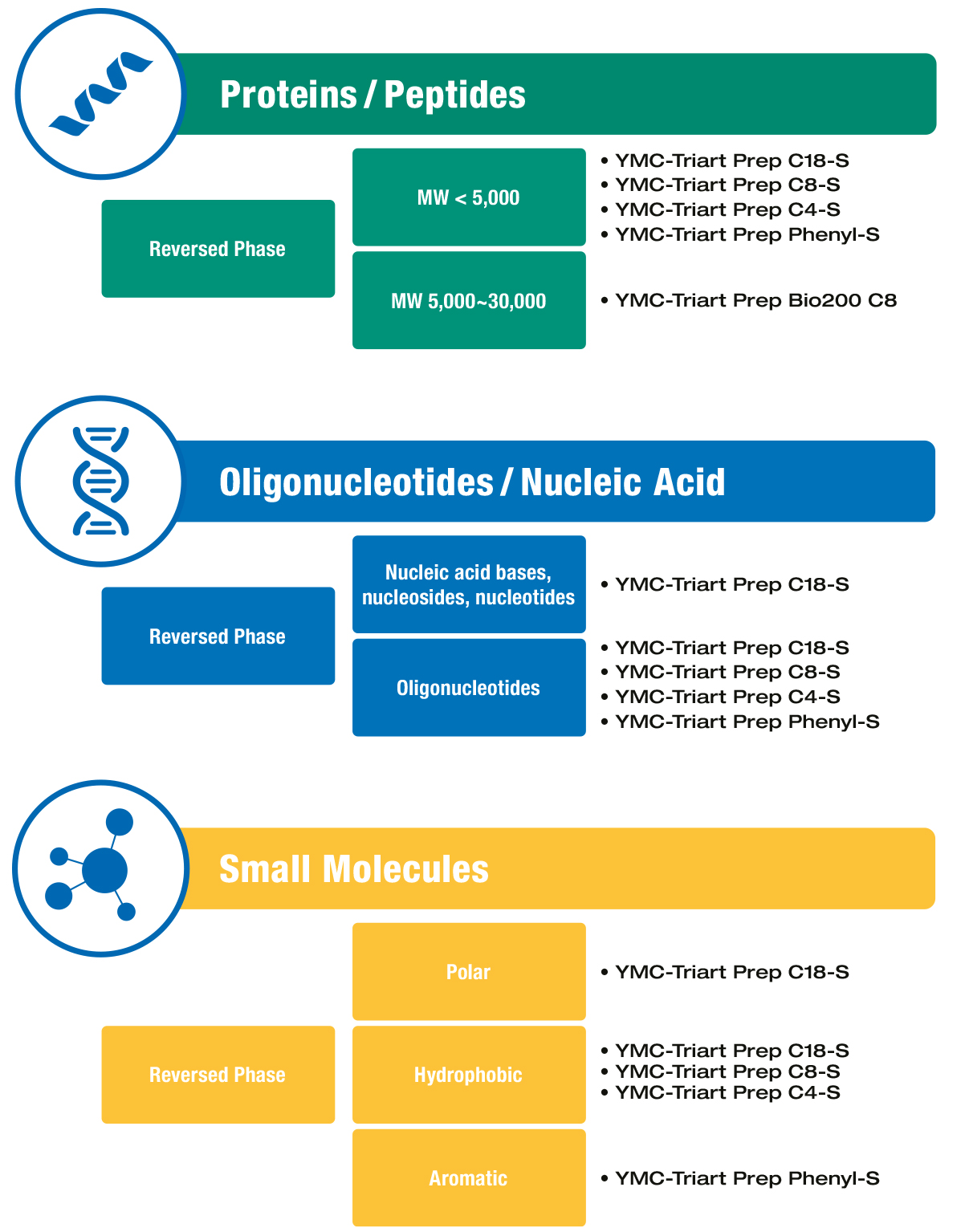

Challenge
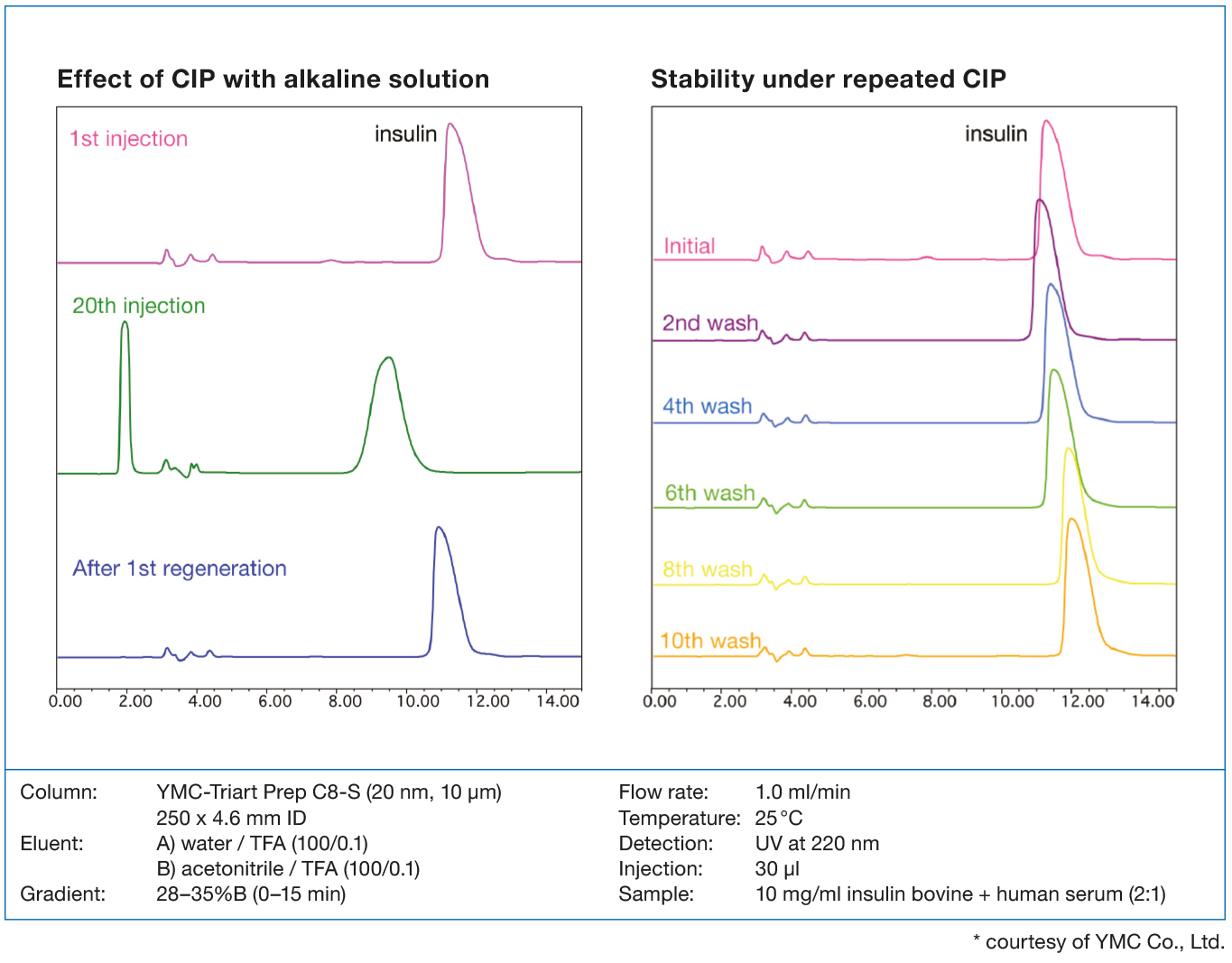
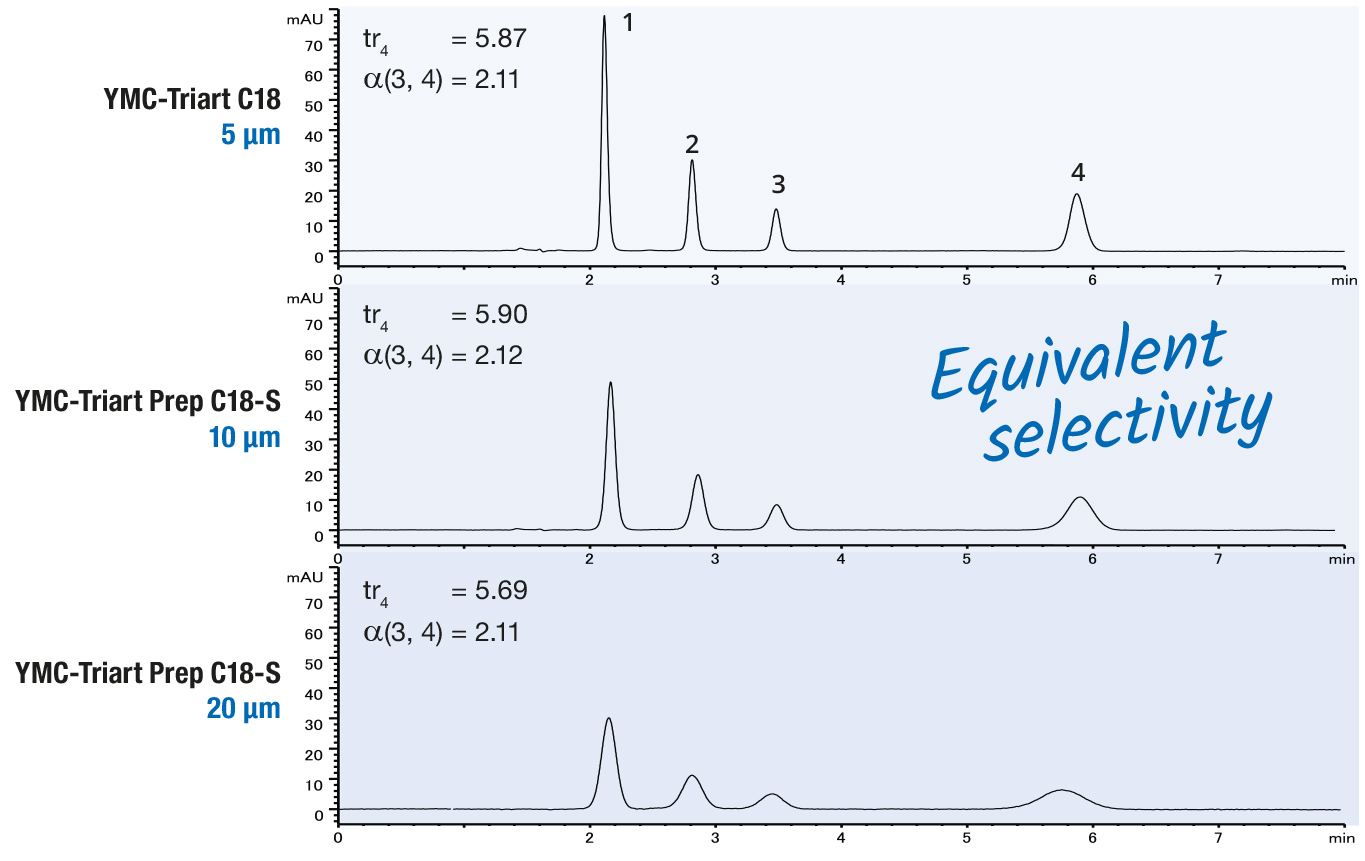
Silica materials are unsuitable for alkaline wash conditions, because of their poor stability at high pH.




| Title | Document Type | Language | Download |
 Excellent alkaline CIP-stability of YMC-Triart Prep leads to long lifetime Excellent alkaline CIP-stability of YMC-Triart Prep leads to long lifetime |
Application Note | EN | |
 Increased Productivity with YMC-Triart Prep Increased Productivity with YMC-Triart Prep |
Application Note | EN | |
 Next generation RP phase for enhanced insulin purification - Based on clients’ real-life data Next generation RP phase for enhanced insulin purification - Based on clients’ real-life data |
Application Note | EN | |
 Oligonucleotide Separations Using Ion-Pairing Reversed Phase Liquid Chromatography Oligonucleotide Separations Using Ion-Pairing Reversed Phase Liquid Chromatography |
Application Note | EN | |
 Purification of Peptides with Full Flexibility Purification of Peptides with Full Flexibility |
Application Note | EN | |
 Purification of polar compounds with YMC-Triart Prep C18-S Purification of polar compounds with YMC-Triart Prep C18-S |
Application Note | EN | |
 The Benefit of Scalability of YMC-Triart Prep in Oligonucleotide Separations The Benefit of Scalability of YMC-Triart Prep in Oligonucleotide Separations |
Application Note | EN | |
 YMC-Triart Prep Packing Materials YMC-Triart Prep Packing Materials |
Brochure | EN | |
 YMC-Triart Prep C4-S Care and Use YMC-Triart Prep C4-S Care and Use |
Care and Use Instruction | EN | |
 YMC-Triart Prep Phenyl-S Care and Use YMC-Triart Prep Phenyl-S Care and Use |
Care and Use Instruction | EN | |
 YMC-Triart Prep Materials Care and Use YMC-Triart Prep Materials Care and Use |
Care and Use Instructions | EN | |
 Are the results from my screening studies always correct? Are the results from my screening studies always correct? |
Expert Tip | EN | |
 Purification of peptides - Increasing the purity and recovery by salt addition Purification of peptides - Increasing the purity and recovery by salt addition |
Expert Tip | EN | |
 High Resolution Purifications with 7 µm YMC-Triart Prep C18-S High Resolution Purifications with 7 µm YMC-Triart Prep C18-S |
Flyer | EN | |
 Selection Tool for the YMC-Triart Prep Family - First Choice in Preparative LC Selection Tool for the YMC-Triart Prep Family - First Choice in Preparative LC |
Flyer | EN | |
 YMC-Triart Prep Phenyl-S YMC-Triart Prep Phenyl-S |
Flyer | EN | |
 Your Complete Screening Set-up with YMC-Triart Prep Your Complete Screening Set-up with YMC-Triart Prep |
Flyer | EN | |
 Packen von Silika- und Hybrid-Silika Stationärphasen in DAC-Säulen Packen von Silika- und Hybrid-Silika Stationärphasen in DAC-Säulen |
Overview | DE | |
 Packen von Silika- und Hybrid-Silika Stationärphasen in Glassäulen Packen von Silika- und Hybrid-Silika Stationärphasen in Glassäulen |
Overview | DE | |
 Packing Silica and Hybrid-Silica Stationary Phases into DAC Columns Packing Silica and Hybrid-Silica Stationary Phases into DAC Columns |
Overview | EN | |
 Packing Silica and Hybrid-Silica Stationary Phases into Glass Columns Packing Silica and Hybrid-Silica Stationary Phases into Glass Columns |
Overview | EN | |
 YMC Bulk Packing Materials for RP-, NP- and Chiral Prep. LC YMC Bulk Packing Materials for RP-, NP- and Chiral Prep. LC |
Overview | EN | |
 YMC Resins for Preparative Bioseparations YMC Resins for Preparative Bioseparations |
Overview | EN | |
 Novel hybrid reversed phase packing material for high productivity of insulin purification Novel hybrid reversed phase packing material for high productivity of insulin purification |
Poster | EN | |
 Oligonucleotide purification using anion exchange (AEX) and ion-pair reversed phase chromatography (IP-RP LC) Oligonucleotide purification using anion exchange (AEX) and ion-pair reversed phase chromatography (IP-RP LC) |
Poster | EN | |
 Purification method development for Liraglutide Purification method development for Liraglutide |
Poster | EN | |
 The importance of mechanical stability in process-scale chromatography The importance of mechanical stability in process-scale chromatography |
Poster | EN | |
 How to reduce the costs for preparative LC processes How to reduce the costs for preparative LC processes |
Product Information | EN | |
 Next generation RP phase for peptide purification: YMC-Triart Prep Bio200 C8 Next generation RP phase for peptide purification: YMC-Triart Prep Bio200 C8 |
Product Information | EN | |
 Desalting/Resalting using a RP column Desalting/Resalting using a RP column |
Technical Note | EN | |
 Linear Scale-Up in Preparative LC Method Development Linear Scale-Up in Preparative LC Method Development |
Technical Note | EN | |
 Loadability and productivity for prep LC processes Loadability and productivity for prep LC processes |
Technical Note | EN | |
 Maximise the Productivity of Your Process with the Correct Pore Selection Maximise the Productivity of Your Process with the Correct Pore Selection |
Technical Note | EN | |
 Packing Properties of YMC-Triart Prep Packing Properties of YMC-Triart Prep |
Technical Note | EN | |
 Same but Different: About the Selectivities of Phenyl Stationary Phases Same but Different: About the Selectivities of Phenyl Stationary Phases |
Technical Note | EN | |
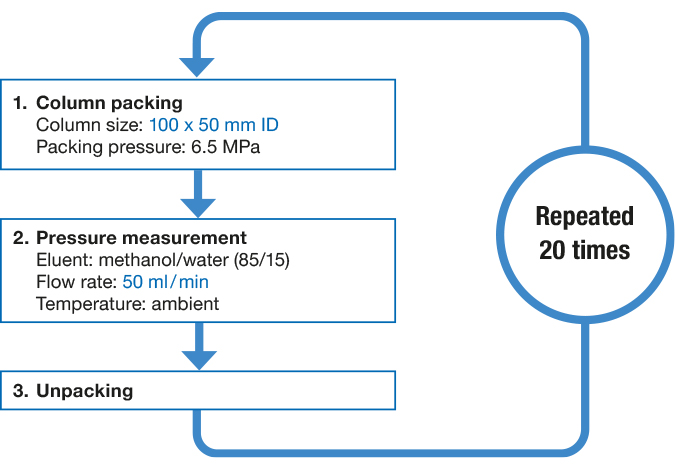
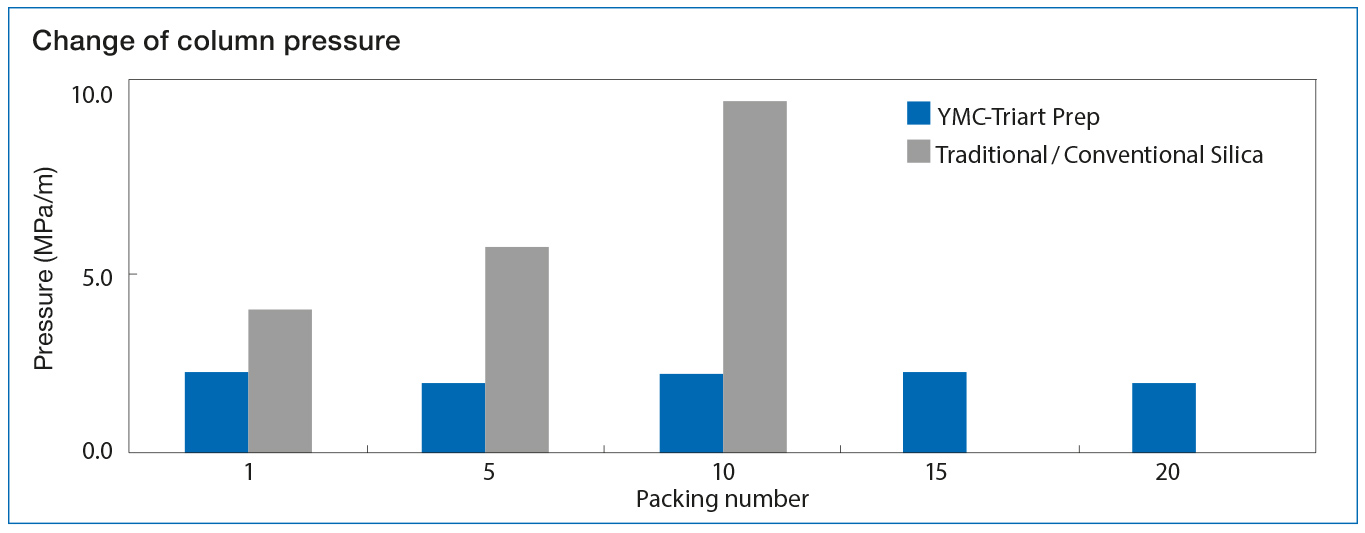
 Sustainability and Mechanical Stability of YMC-Triart Prep Sustainability and Mechanical Stability of YMC-Triart Prep |
Technical Note | EN | |
 Analysis and Purification of Oligonucleotides by Anion Exchange and Ion-Pairing Reversed Phase Chromatography Analysis and Purification of Oligonucleotides by Anion Exchange and Ion-Pairing Reversed Phase Chromatography |
Whitepaper | EN |

Please contact YMC for pricing or specific solutions for your purification process.
Julia Bartmann
Product Manager Bulk Media
ph. +49 2064 427-260
Particle Size | YMC-Triart Prep C18-S | YMC-Triart Prep C8-S | YMC-Triart Prep Bio200 C8 | YMC-Triart Prep Phenyl-S | YMC-Triart Prep C4-S |
| 7 µm | TAS12S07 | ||||
| 10 µm | TAS12S11 | TOS12S11 | TOB20S11 | TPS12S11 | TBS12S11 |
| 15 µm | TAS12S16 | TOS12S16 | |||
| 20 µm | TAS12S21 | TOS12S21 |
Other particle and pore size combinations are available on request.
Example of existing customised materials:
TOS20S11, YMC-Triart Prep C8-S, 20 nm / 200 Å, 10 µm
Typical pack sizes:
Laboratory scale: smallest amount is 100 grams up to 4 kg PE bottle
Industrial scale: more than 4 kg in double lined PE bags inside metal drums (10 or 25 kg drums)